07/08/2024
07/08/2024
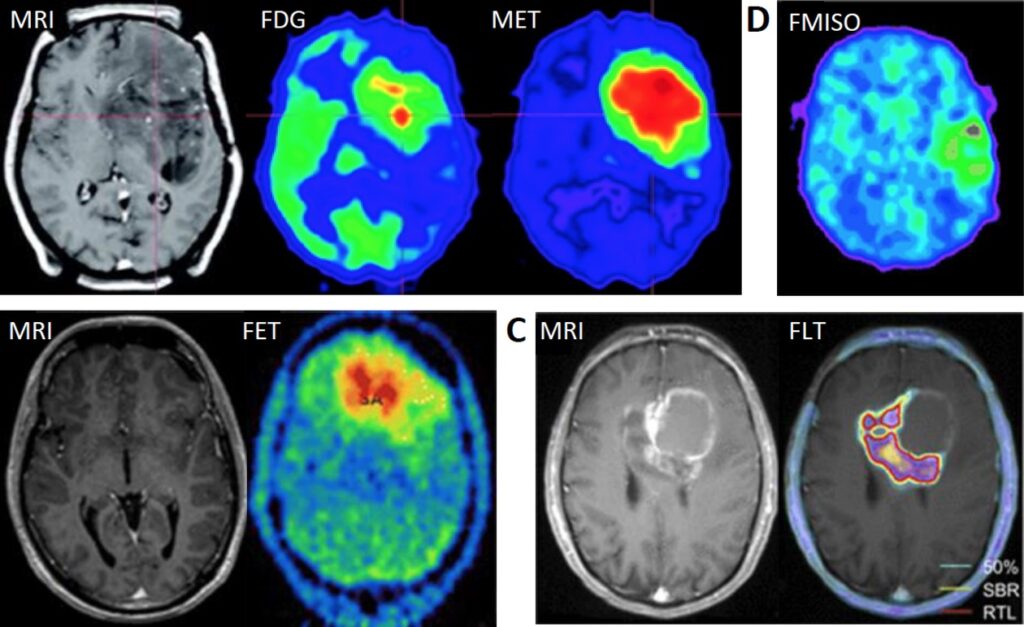
NEW YORK, Aug 7: Servier Pharmaceuticals announced on Tuesday that the U.S. Food and Drug Administration (FDA) has approved its treatment, Voranigo, for a rare type of brain tumor. This approval marks the first time a drug has been granted U.S. approval for this specific condition.
Voranigo is designed to treat Grade 2 IDH-mutant glioma, a form of brain cancer that occurs in patients who have previously undergone surgery. Gliomas, which can impair normal brain function, are traditionally treated through tumor removal. Grade 2 IDH-mutant glioma is linked to mutations in the isocitrate dehydrogenase (IDH) gene family.
The FDA's approval was based on results from a late-stage clinical trial. Patients receiving Voranigo demonstrated a progression-free survival rate of 27.7 months, significantly longer than the 11.1 months observed in the placebo group.
In the U.S., approximately 0.7 cases of IDH-mutant glioma occur per 100,000 people.
Following the approval, Servier and Royalty Pharma will make milestone payments totaling up to $1.1 billion to Agios Pharmaceuticals. This follows Agios's sale of its oncology business to Servier in 2021, for which it received $1.8 billion in upfront cash. Agios is also entitled to a $200 million milestone payment upon FDA approval of Voranigo and will earn 15% royalties on the drug's potential net sales in the U.S.
Earlier this year, Agios sold a portion of its Voranigo royalty rights to Royalty Pharma. As part of this agreement, the FDA's approval triggers a $905 million payment to Agios.


